|
 |
 |
|
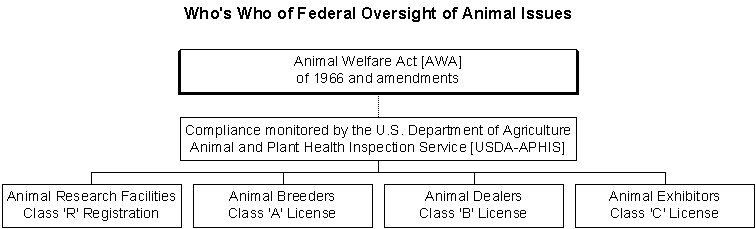
The Animal Welfare Act [often referred to as "The AWA", or "The Act"] regulates the purchase, care, treatment and transportation of animals used for exhibition, sold as pets, or used in biomedical research laboratories. At the present time, the Act does not cover animals used in commonly accepted agricultural practices, laboratory-bred rats, mice nor birds. The AWA initially covered only the care and transportation of animals and made provisions to prevent the sale of stolen animals. The first version of the AWA (called the Laboratory Animal Welfare Act) was passed in 1966 in response to public criticisms about pound seizure and bunching. ["Pound seizure" is the practice of selling (or donating) animals surrendered to a shelter to biomedical research facilities. "Bunching" is theft of pet dogs and cats for the purpose of selling them to be used in biomedical research.] Since 1966, there have been several amendments to the AWA which provide more protection for animals. For example, in 1985 an amendment required "exercise of dogs" and "psychological well-being of primates". Click here for more information about the compliance of the requirement for "psychological well-being of primates". In 1990, the Act was amended to define a minimum holding period (five days) for animals held in shelters who were to be sold to research facilities. The most recent amendment which is known as 'The Farm Bill' was introduced by Jesse Helms and signed by President Bush in 2002. 'The Farm Bill' is intended to permanently excludes birds, mice, and rats from AWA protection. The United States Department of Agriculture's Animal and Plant Health Inspection Service, Animal Care Division [USDA-APHIS/AC] monitors compliance with the Animal Welfare Act [AWA] of l966 and its amendments. APHIS inspectors are supposed to conduct unannounced inspections at least once a year at licensed and registered facilities. Click here for a listing of APHIS licensees/registrants When minor violations of the AWA are found and cited, the licensed facility is given the opportunity to correct the problem within a determined amount of time. Major violations can lead to substantial fines and suspension of registration or license. Many argue that the animal protection provisions in the AWA are too vague or simply inadequate. For example, as noted above, rats, mice and birds (animals comprising more than 90% of the animals used in research) are not afforded any AWA protection. Many of the AWA provisions are written in a manner which leaves them subject to interpretation; such as the provision requiring "adequate lighting". Others are critical of APHIS enforcement of the AWA. Some common complaints about licensing of dealers, breeders and exhibitors are as follows: *APHIS licenses are too easily obtained. [Over 2,500 permits were issued in year 2001 alone.] *Permits are issued with minimum requirements on pre-licensing inspections. *APHIS inspectors are not necessarily trained nor qualified in the field of exotic/wild animal care. *Even in the worst animal neglect/abuse situations, there is little to no accountability required and often a lack of follow-up or due diligence by APHIS. *APHIS very rarely confiscates animals. In some cases they will revoke a permit, leaving the owner to place the animals with other breeders, dealers, exhibitors. As such, many animals each year are recycled back into substandard facilities. *When a licensee's permit is revoked there is nothing that prohibits a family member of that former licensee to obtain a license which allows the individual whose license has been revoked to conduct 'business as usual'. *In many -- if not most -- instances, when the permit holder is found guilty and fined for noncompliance, APHIS waives the fines. *When displaced animals are sent to credible, above-board sanctuaries no financial assistance is given towards enclosure costs and the costs associated with providing the animals with lifetime care. Additionally, the application for licensing of dealers, breeders and exhibitors does not require the following: *Proof of ownership of property; Some Solutions for Making Improvements in Animal Protection *Increase number of APHIS inspectors charged with monitoring compliance. *The agency should provide extensive species-specific training to APHIS inspectors. *The agency should provide support and encouragement to APHIS inspectors who are dealing with enforcement problems. *Record-keeping about each animal's disposition, from birth throughout any transfer(s) should be centralized. APHIS should create this tracking database and this goal can be achieved by requiring each animal to be permanently marked for identification (micro-chip, tattoo, tag) and registered at birth. *The agency should disallow any licensee who has had a permit revoked to operate under someone else's permit. Some Solutions for Making Improvements in Enforcement USDA-APHIS should do the following: *Institute a cessation of permit issuance until the agency's is better staffed with inspectors and those inspectors are properly trained. *Make recommendations to Congress to upgrade current AWA minimum requirements based on the abusive and neglectful situations they encounter in the course of their duties. *Issue a check-list and specific guidelines pertaining to AWA requirements to APHIS inspectors and require them to follow one set of procedures. *Follow up on all non-compliant items by the date stipulated in the initial inspection report. *Conduct inspections at more frequent intervals at facilities that show continual problems. *Assist the animal protection community with getting a definition for legitimate/credible sanctuaries included in the AWA and help set-up a separate licensing category for credible sanctuaries using agreed-upon standards as a guideline. *Accept responsibility for the outcome of displaced animals when permits are revoked or when the licensed facility goes out of business. *APHIS should begin collecting fines imposed and using the fines to cover costs associated with relocating animals in credible sanctuaries. Note The entire AWA is available for reading or downloading from the internet at this URL http://www.nal.usda.gov/awic/legislat/usdaleg1.htm AWA regulations on the humane handling, care, treatment, and transportation of nonhuman primates are contained in 9 CFR part 3, subpart D |
|
|
|
|